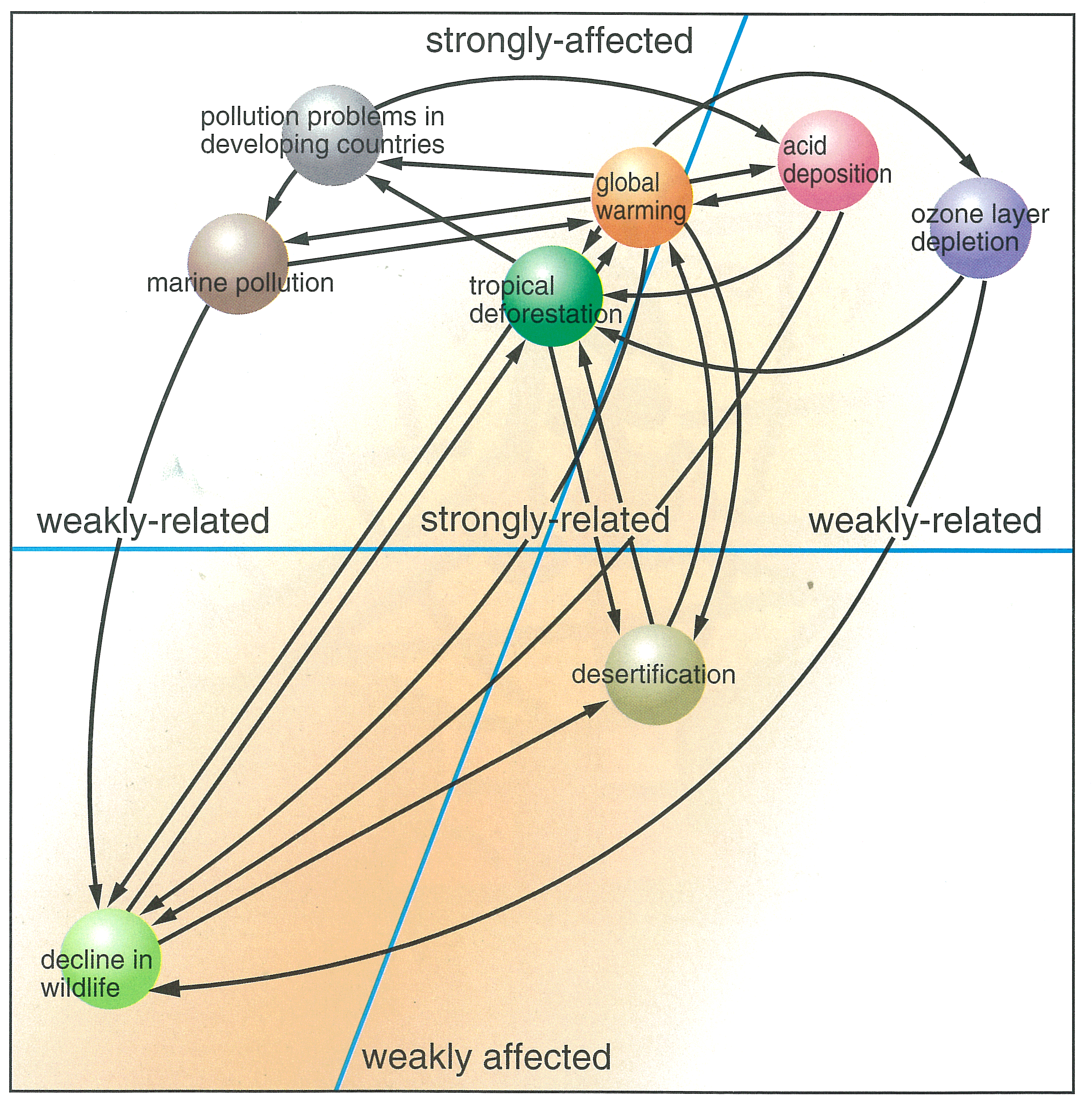
Have you heard of the term "global environmental issues" ? Global environmental issues include ozone layer depletion, global warming, acid deposition, tropical deforestation, desertification, pollution problems in developing countries, endangered species, marine pollution, and transboundary movement of hazardous wastes.
Why are they called global environmental issues ? It's because their impacts and damages affect not only the countries that caused the problems, but go beyond their national boundaries and can reach a global scale. It's also because these are problems which require international efforts for solution.
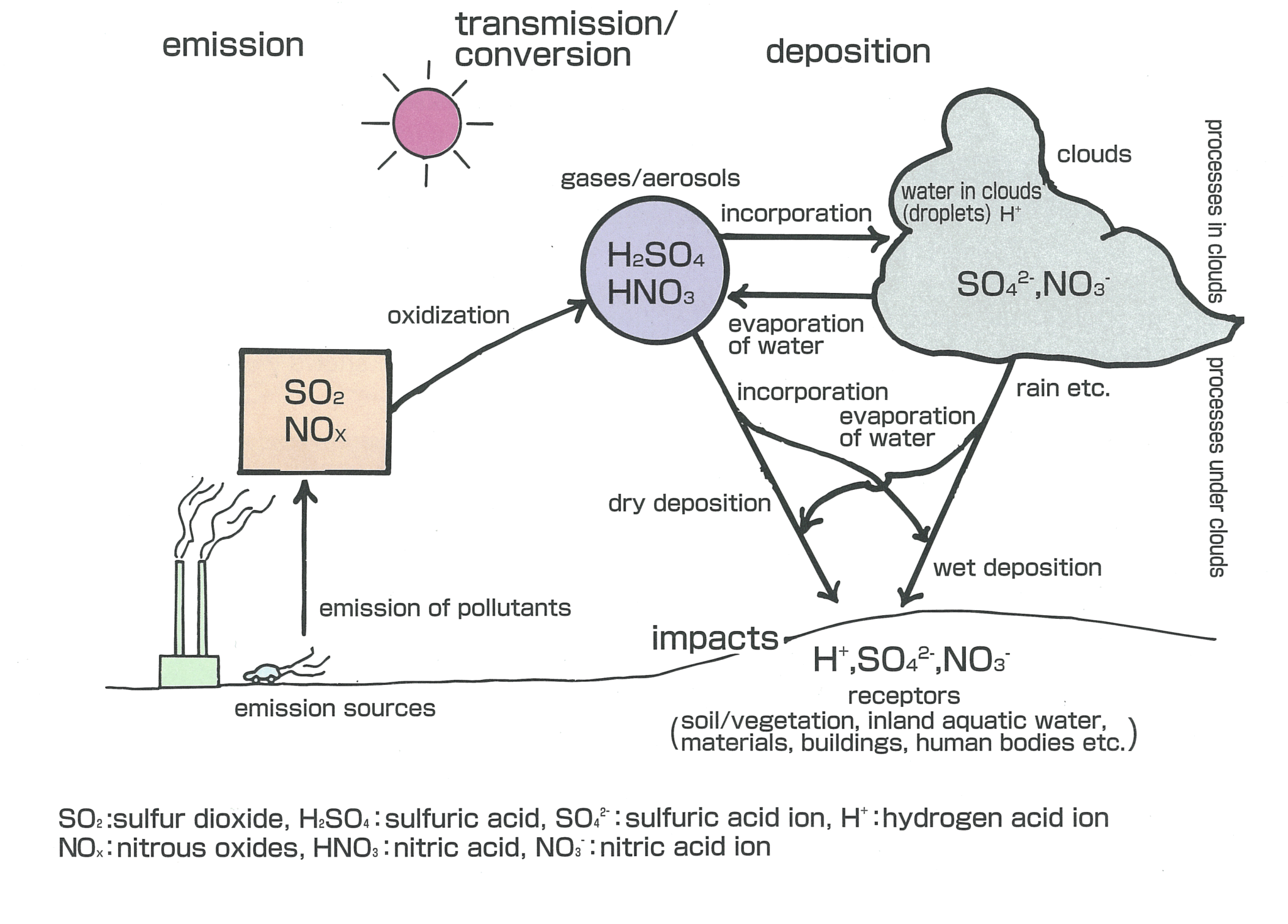
These problems are inter-related in a very complicated manner. For instance, burning of fossil fuels, such as oil and coal, increases emissions of carbon dioxide that cause global warming, and at the same time, emissions of sulfur dioxide and nitrogen oxides, the major pollutants causing acid deposition. These problems also lead to deforestation and threats to wildlife. In industrialized countries, people are producing, consuming and disposing of huge amount of products. Significant amount of resources and energy are required for the production, consumption and disposal of such products. This is one of the major causes of global environmental problems such as acid deposition and global warming.